N-BROMO SUCCINIMIDE CAS No. 128-08-5
General Description
N-Bromo Succinamide (NBS) is a popular bromination reagent. It is a versatile source of bromine for reactions such as electrophilic additions and radical substitution.

Advantages over other reagents:
The easy removal of Succinamide after bromination.
The possibility to fine tune its reactivity and properties via combination with other reagents
It is therefore widely used in the synthesis of advanced fine chemical intermediates, especially in the area of pharmaceuticals.
Applications:
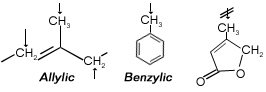
1) Bromination in Allylic and Benzylic positions:
NBS is widely known as its ability to brominate in Allylic and benzlic positions if used in combination with a source of radicals.
Apart from reacting selectively in Allylic positions, it shows remarkable selectivity of Allylic methylene over Allylic methyl.
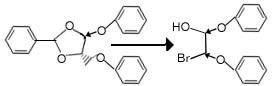
In Benzylic brominations, no HBr is formed which makes in the presence of HBr sensitive functional groups is required. The cleavage of benzylidene acetals is a selective method to remove benzylidene protection of vicinal diols and has found applications in the synthesis of natural products, especially carbohydrates.
2) ά- Bromination of Carbonyl Derivatives:
Carbonyl derivatives can only be attacked if radical intermediates are stabilized, for example by the captodative effect. According to this principle. ά-amino acid derivatives can be ά-brominated.
3) Bromination of aromatic compounds:
Electron rich aromatic compounds can be brominated regioselectively.
4) Bromohydration and Bromolactonisaion:
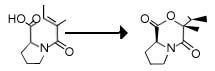
The bromination of alkenes gives, 2-bromohydrines, with selective attack on the most electron-rich double bond. Bromohydrines can be transformed into a variety of useful derivates among them epoxides. Initial attack of bromine to a double bond can be followed by intramolecular attack giving rise to lactones, cyclic ethers etc.
Combination with other reagents:
The bromination properties of NBS can be fine-tuned via combination with other reagents.
- NBS/HBr is used to convert acid chlorides into ά -bromo-acid chlorides.
- Combination with nucleophiles: In the course of addition to a double bond a variety of nucleophiles can be used: addition of azide ions yields to bromo azides; addition of fluoride ions yields to bromo fluorides.
- NBS/DMF is an effective blend for the selective monobromination of electron-rich aromatic compounds.
- NBS/Dimethyl sulfide converts Allylic and Benzylic alcohols into the corresponding bromides, even in the presence of sensitive functional groups.
- The system can also be used to oxidize other, both for the selective monobromination of electron-rich aromatic compounds.
- NBS/Dimethyl sulfide converts Allylic and Benzylic alcohols into the corresponding bromides, even in the presence of sensitive functional groups.
The system can also be used to oxidize other, both primary and secondary alcohols, to the corresponding carbonyl compounds.
Handling:
Packing
Tariff No.
EINECS No.
TSCA
MITI
Toxicity
Safety recommendations
: PE drums of 25kg & 50kg net
: 292519
: 2048772
: not listed
: 5 - 121
: LD50 (rat, oral): 2000mg/kg
: R36, R37, R38;
S26, S28, S36, S43


